Calidad
Quality
Quality in the healthcare sector is something that cannot be improvised
Products intended for healthcare and well-being of people must comply with the strictest controls to guarantee their correct performance, offer the healthcare professional optimal performance, and the patient the greatest possible well-being.
At Telic we have been making quality the engine of our organization for 40 years, transferring it to all operational areas.
We have the ISO 13485 certificate for the quality management system for medical devices and the certificate of compliance with good manufacturing practices (GMP / GMP) for cosmetic products, equivalent to ISO 22716, issued by the AEMPS. All our products are subjected to exhaustive verification processes so that they reach the market in the best possible conditions. Some of them are checked one by one, since the life of a person can depend on their correct performance in an instant.

Quality in medical devices
ISO 13485:2016 standard focuses on the importance of the life cycle of a medical device, including its design, development, production, storage, distribution, installation, maintenance and final dismantling. This fact implies that its fulfillment is a firm commitment that relates all the people who collaborate in our organization, involved in any process, under a common objective. It is, in short, a team effort from start to finish.
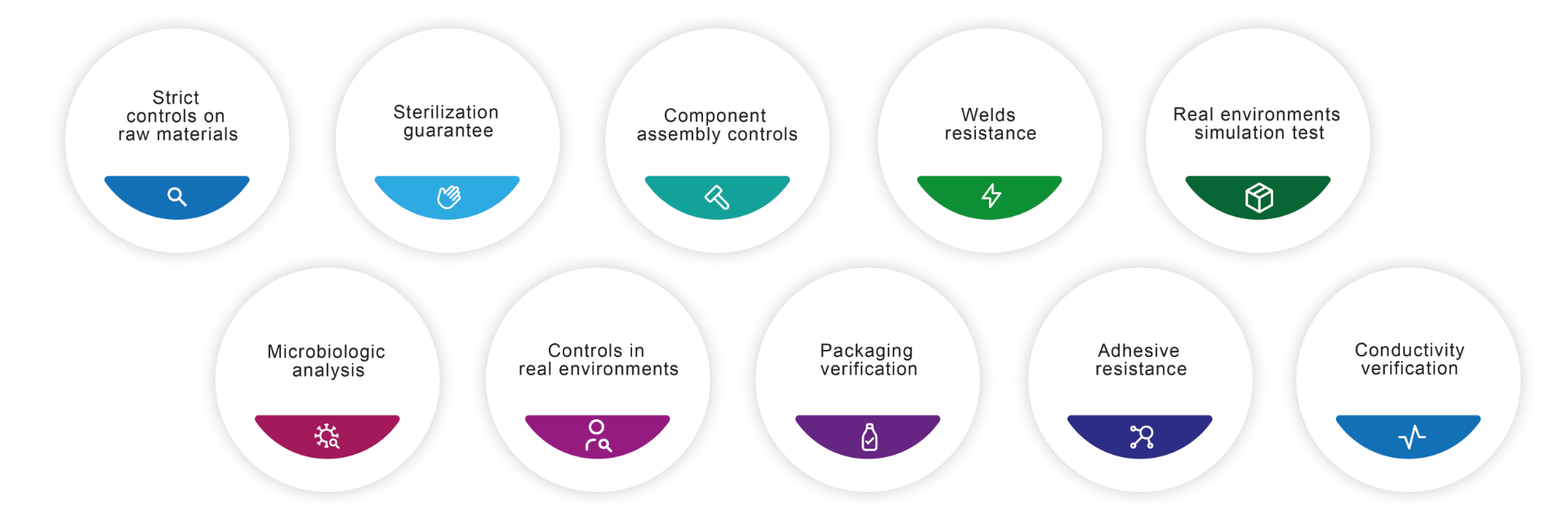
Telic is synonymous with guarantee and service. This is demonstrated by the fact that our consumables are found in hospitals all over the world and that the trust in our organization continues to grow. We expose each and every one of our products to strict quality controls, these are some of the examples of the analysis we carry out.
Quality in cosmetics products
Our experience in the sector and the demand that the development of our productive and commercial activity in the health field implies, has led us to the development and manufacture of cosmetic products of the highest quality.
Thanks to our R&D department, we develop and manufacture our own formulas in our facilities, and in 2021 the AEMPS granted us the certificate of compliance with good manufacturing practices (GMP), which is equivalent to ISO 22716 for the manufacture of cosmetic products.
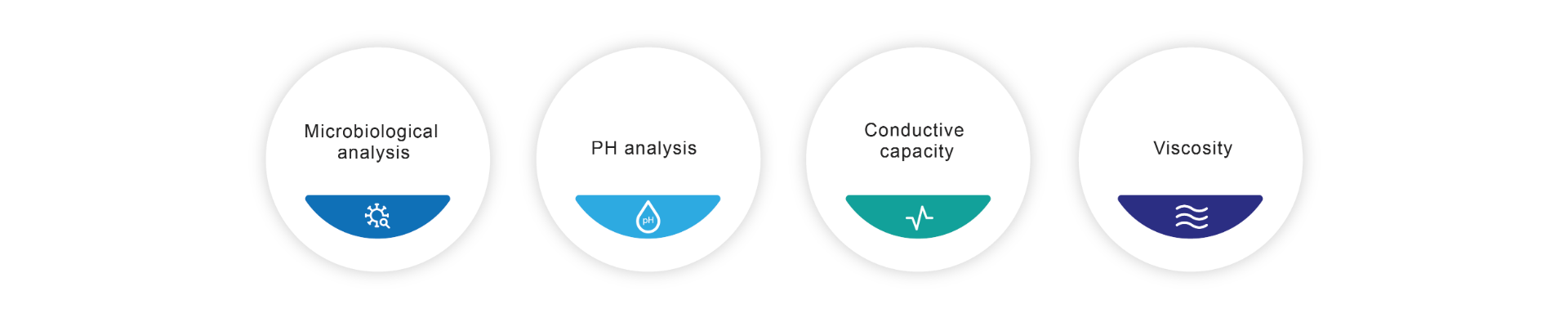
All our cosmetic products, depending on their classification, are subjected to different tests to verify their safety, which include, among others, microbiological tests, pH tests, conductive capacity and viscosity.
We subject each and every one of our cosmetic products to strict quality controls, these are some of the examples of the analysis we carry out

 Español
Español Français
Français